https://www.nature.com/articles/d41586-020-01801-y
Forkhead BioTherapeutics is a spin-off from Columbia University in New York City.
You can’t live without insulin. When people with type 1 diabetes lose their ability to produce this hormone, they need to inject synthetic insulin multiple times a day and closely monitor their blood sugar levels. Managing the disease is difficult, and people with diabetes are at high risk of both emergency hospitalization and serious long-term conditions such as cardiovascular disease and blindness.
Read more about The Spinoff Prize
What if there were a pill that could restart insulin production in people with type 1 diabetes? That’s the goal of Forkhead BioTherapeutics in New York City, which hopes to begin clinical trials in 2022. The pill would not be insulin in some cleverly packaged form, but instead be designed to persuade cells in the intestine that usually produce a hormone known as serotonin to automatically produce the insulin people need, in the right amounts, at the right time.
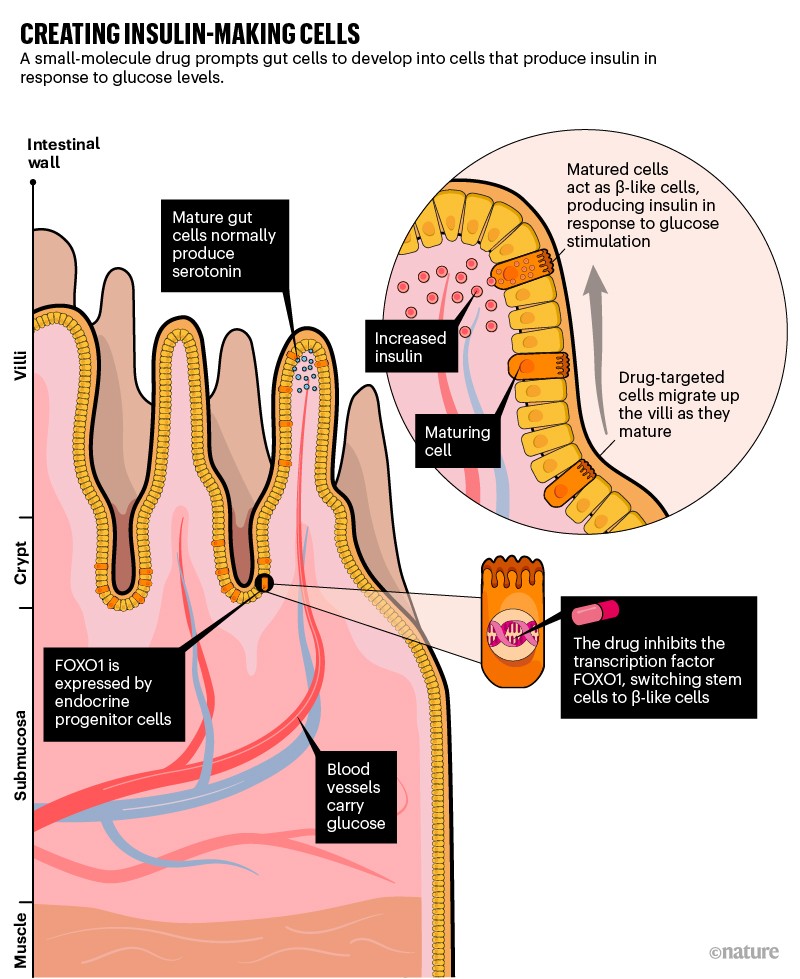
This unusual approach comes from the lab of Domenico Accili, an endocrinologist at Columbia University in New York City. For decades Accili has studied the FOXO family of transcription factor proteins, which are master regulators of many genes. He found that when mice were engineered without the ability to express one such protein, called FOXO1, cells in their gut wall produced insulin (see ‘Creating insulin-making cells’).
“At first glance, this seems pretty odd,” acknowledges Charles Queenan, Forkhead’s chief executive. “But if you ask a developmental biologist what cell in the body is most closely related to the β cell in the pancreas that makes insulin, and is lost in type 1 diabetes, it would be the hormone-producing enteroendocrine cell in the gut that we’re targeting.”
“We’re flipping a switch,” he explains. “Instead of an enteroendocrine cell that makes one hormone, you flip the switch and it ends up terminally differentiating into a cell that makes insulin.”
Accili and his colleagues have subsequently confirmed the process in human organoids (3D tissue models generated with stem-cell techniques), raising the possibility that inhibiting FOXO1 proteins could help in treating diabetes1.
Part of Nature Outlook: The Spinoff Prize 2020
Transcription factors are notoriously difficult to target with small-molecule oral drugs, says Hua Lin, chief scientific officer at Forkhead. However, in 2017, Accili’s group identified several classes of compounds that inhibit FOXO1 in human cells2.
That put the pieces in place for a commercial start-up, says Queenan, a biotech entrepreneur whose daughter was diagnosed with type 1 diabetes at the age of three. He founded Forkhead with Accili and Lin in 2017.
“New technologies with the prospect of replacing insulin, as this does, have enormous potential,” he says. In addition to people with type 1 diabetes, many with the type 2 form of the disease end up needing insulin, too. The global annual insulin market is estimated to be around US$30 billion; a pill-based alternative could offer Forkhead a large and steady flow of revenue.
Selecting a compound
Forkhead now has five employees and invests heavily in research both at Columbia and through a network of contract-research organizations. The company hopes to pick a lead compound either late this year or early next. “Part of our strategy is to develop inhibitors that only inhibit FOXO1 in the gut — limiting systemic circulation of the drug so that it doesn’t hit FOXO1 all over the body,” says Lin. Once a compound is picked, Forkhead expects to test it in a large animal model, probably a non-human primate, during 2021.
Assuming all goes well, the company will proceed to a phase I clinical trial, probably involving healthy volunteers and people with type 1 diabetes. This trial could be small and relatively quick, and provide both safety information and an early indication on how effectively the pill stimulates insulin production and helps the body to maintain blood glucose levels in a normal range, Lin says.
One question the researchers are unsure of is whether the insulin-producing gut cells might pay too much attention to glucose levels in the gut and not enough to those in the blood. It is also possible that the autoimmune reaction responsible for disabling insulin-producing cells in the pancreas in type 1 diabetes will attack insulin-producing cells in the gut. But, because cells in the gut, unlike those in the pancreas, are quickly replenished, that might not spell disaster for the therapy.
Many other labs and a few drug companies have competing projects for insulin-producing cells. The most advanced efforts aim to generate the cells in vitro using stem-cell techniques, and transplant them inside capsules that guard against immune attack. Even if such efforts are successful, Queenan contends, Forkhead’s pill will be more convenient for people than a surgical implant that might provoke immune reactions.
“Forkhead is taking a very interesting approach, different from the stem-cell differentiation-based therapeutics that everyone else is working on,” says Qiao Zhou, a developmental biologist at Weill Cornell Medicine Graduate School of Medical Sciences in New York City and an expert in applying regenerative medicine research to insulin-producing cells. “It might actually work.” Zhou is, however, cautious about whether the approach can generate the hundreds of millions of insulin-producing cells needed to fully control the disease. “Also, how safe is inhibiting FOXO1, which is expressed all over the body?” he asks.
If the clinical trial succeeds, Forkhead will probably become a candidate for acquisition by one of the major insulin suppliers, because the technology would be disruptive to their business. A partnership with a large drug firm could also provide resources for a product for type 2 diabetes, the approval of which would require extremely long and expensive trials, Lin says.
Adam Irvine, a specialist in commercializing academic intellectual property based in Edinburgh, UK, and one of the judges of the Nature Research Spin-off Prize, rates the Forkhead leadership team and its drug-development expertise highly. He also applauds the ambition to treat type 1 diabetes in a unique way: “It’s like they are doing deep-sea drilling, while other people are still trying to drill on land.”